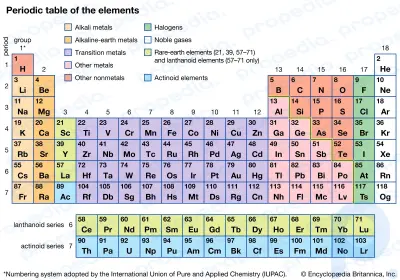
What is the definition of an alkali metal?
The alkali metals are six chemical elements in Group 1, the leftmost column in the periodic table. They are lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). (Like the other elements in Group 1, hydrogen (H) has one electron in its outermost shell, but it is not classed as an alkali metal since it is not a metal but a gas at room temperature.)
Periodic Table of the ElementsBrush up on the periodic table of elements.Why are they called the alkali metals?
The alkali metals are so named because when they react with water they form alkalies. Alkalies are hydroxide compounds of these elements, such as sodium hydroxide and potassium hydroxide. Alkalies are very strong bases that are caustic. Lye, for example, is sodium hydroxide. Alkalies react with acids to form salts.
baseRead more about bases.What are some properties of the alkali metals?
The alkali metals have low melting points. Lithium melts at 180.5 °C (356.9 °F); cesium melts at just 28.4 °C (83.1 °F). These elements are also excellent conductors of heat and electricity. The alkali metals are very reactive and so are usually found in compounds with other elements, such as salt (sodium chloride, NaCl) and potassium chloride (KCl).
Read more below: General properties of the groupWhat is the most common alkali metal?
The most common alkali metal is sodium, which is 2.8 percent of Earth’s crust. The most common sodium compound is sodium chloride (NaCl), salt. The next most common is potassium, which is 2.6 percent of Earth’s crust. The other alkali metals are much rarer. Rubidium, lithium, and cesium are 0.01, 0.002, and 0.0007 percent of Earth’s crust, respectively. Francium is radioactive, and only minute amounts of it exist in nature.
sodiumLearn more about sodium.alkali metal, any of the six chemical elements that make up Group 1 (Ia) of the periodic table—namely, lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). The alkali metals are so called because reaction with water forms alkalies (i.e., strong bases capable of neutralizing acids). Sodium and potassium are the sixth and seventh most abundant of the elements, constituting, respectively, 2.6 and 2.4 percent of Earth’s crust. The other alkali metals are considerably more rare, with rubidium, lithium, and cesium, respectively, forming 0.03, 0.007, and 0.0007 percent of Earth’s crust. Francium, a natural radioactive isotope, is very rare and was not discovered until 1939.
The alkali metals are so reactive that they are generally found in nature combined with other elements. Simple minerals, such as halite (sodium chloride, NaCl), sylvite (potassium chloride, KCl), and carnallite (a potassium-magnesium chloride, KCl · MgCl2· 6H2O), are soluble in water and therefore are easily extracted and purified. More complex, water-insoluble minerals are, however, far more abundant in Earth’s crust. A very dilute gas of atomic sodium (about 1,000 atoms per cubic cm [about 16,000 atoms per cubic inch]) is produced in Earth’s mesosphere (altitude about 90 km [60 miles]) by ablation of meteors. Subsequent reaction of sodium with ozone and atomic oxygen produces excited sodium atoms that emit the light we see as the “tail” of a meteor as well as the more diffuse atmospheric nightglow. Smaller amounts of lithium and potassium are also present.
The alkali metals have the silver-like lustre, high ductility, and excellent conductivity of electricity and heat generally associated with metals. Lithium is the lightest metallic element. The alkali metals have low melting points, ranging from a high of 179 °C (354 °F) for lithium to a low of 28.5 °C (83.3 °F) for cesium. Alloys of alkali metals exist that melt as low as −78 °C (−109 °F).

The alkali metals react readily with atmospheric oxygen and water vapour. (Lithium also reacts with nitrogen.) They react vigorously, and often violently, with water to release hydrogen and form strong caustic solutions. Most common nonmetallic substances such as halogens, halogen acids, sulfur, and phosphorus react with the alkali metals. The alkali metals themselves react with many organic compounds, particularly those containing a halogen or a readily replaceable hydrogen atom.
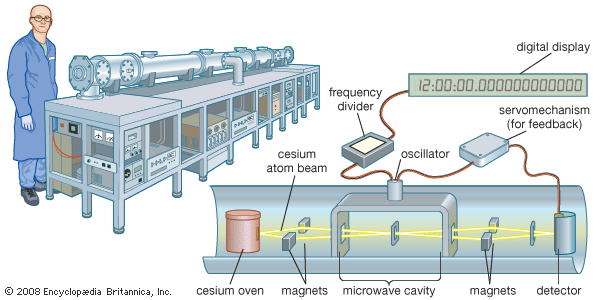
Sodium is by far the most important alkali metal in terms of industrial use. The metal is employed in the reduction of organic compounds and in the preparation of many commercial compounds. As a free metal, it is used as a heat-transfer fluid in some nuclear reactors. Hundreds of thousands of tons of commercial compounds that contain sodium are used annually, including common salt (NaCl), baking soda (NaHCO3), sodium carbonate (Na2CO3), and caustic soda (NaOH). Potassium has considerably less use than sodium as a free metal. Potassium salts, however, are consumed in considerable tonnages in the manufacture of fertilizers. Lithium metal is used in certain light-metal alloys and as a reactant in organic syntheses. An important use of lithium is in the construction of lightweight batteries. Primary lithium batteries (not rechargeable) are widely used in many devices such as cameras, cellular telephones, and pacemakers. Rechargeable lithium storage batteries that could be suitable for vehicle propulsion or energy storage are the subject of intensive research. Rubidium and cesium and their compounds have limited use, but cesium metal vapour is used in atomic clocks, which are so accurate that they are used as time standards.
History
Alkali metal salts were known to the ancients. The Old Testament refers to a salt called neter (sodium carbonate), which was extracted from the ash of vegetable matter. Saltpetre (potassium nitrate) was used in gunpowder, which was invented in China about the 9th century ad and had been introduced into Europe by the 13th century.
In October 1807 the English chemist Sir Humphry Davy isolated potassium and then sodium. The name sodium is derived from the Italian soda, a term applied in the Middle Ages to all alkalies; potassium comes from the French potasse, a name used for the residue left in the evaporation of aqueous solutions derived from wood ashes.
Lithium was discovered by the Swedish chemist Johan August Arfwedson in 1817 while analyzing the mineral petalite. The name lithium is derived from lithos, the Greek word for “stony.” The element was not isolated in pure form until Davy produced a minute quantity by the electrolysis of lithium chloride.
While the German chemists Robert Bunsen and Gustav Kirchhoff were investigating the mineral waters in the Palatinate in 1860, they obtained a filtrate that was characterized by two lines in the blue region of its spectrum (the light emitted when the sample was inserted into a flame). They suggested the presence of a new alkali element and called it cesium, derived from the Latin caesius, used to designate the blue of the sky. The same researchers, on extracting the alkalies from the mineral lepidolite, separated another solution, which yielded two spectral lines of red colour. They proposed the name rubidium for the element in this solution from the Latin rubidus, which was used for the darkest red colour. Francium was not discovered until 1939 by Marguerite Perey of the Radium Institute in Paris.
In the 19th century the only use for the alkali metals was the employment of sodium as a reagent in the manufacture of aluminum. When the electrolytic process for aluminum purification was established, it appeared that large-scale use of sodium would cease. Subsequent improvements in the electrolytic production of sodium, however, reduced the cost of this element to such an extent that it can be employed economically to manufacture gasoline additives, reagents for chemical industry, herbicides, insecticides, nylon, pharmaceuticals, and reagents for metal refining. The continuous electrolysis of sodium hydroxide, a technique called the Castner process, was replaced in 1926 by the Downs cell process. This process, in which a molten sodium chloride–calcium chloride mixture (to reduce the melting point) is electrolyzed, produces both sodium metal and chlorine.